Still many companies are allowed to use their tests on. Quest Diagnostics on Tuesday said its now.
Sensors Free Full Text Covid 19 Testing And Diagnostics A Review Of Commercialized Technologies For Cost Convenience And Quality Of Tests Html
Pooling is an efficient way to evaluate patients in regions or populations with low prevalence of disease less than 10 positivity.

Is quest diagnostics covid 19 antibody test fda approved. Quest Diagnostics Updated. But the reliability of serology tests in general remains unclear. Quest launches consumer-initiated antibody test for COVID-19.
Weeks after launching their own COVID-19 molecular testing initiatives lab giants Quest Diagnostics and LabCorp have begun offering antibody tests nationwide for the next phase of the coronavirus. Ortho Clinical Diagnostics a global leader of in vitro diagnostics with a rich history of bringing critical tests for infectious diseases to market today announced it is working with Quest Diagnostics NYSE. Visa MC AMEX eCheck Paypal Work time.
Book an appointment and receive an email with your appointment details. FDA may issue an. DGX the worlds leading provider of diagnostic information.
Quest Diagnostics Begins to Perform COVID-19 Antibody Testing. The capacity of US. So I tried it.
I got Quests new COVID-19 antibody test and it was strange Last week Quest Diagnostics announced a new direct-to-consumer test for coronavirus antibodies. But there is now a new route for people to pursue serological testing to detect who has already been exposed to the SARS-CoV-2 virus. DGX the worlds leading provider of diagnostic information services to expand COVID-19 antibody testing to more than 20 Quest laboratories across the US.
LabCorp Monday announced its COVID-19 antibody tests will be a vailable nationwide with no out-of-pocket costs with an order from a healthcare provider. Quest Diagnostics has received emergency use authorization EUA from the US Food and Drug Administration FDA to use pooled specimens in connection with molecular diagnostic testing for COVID-19. The very best prices available today fast delivery.
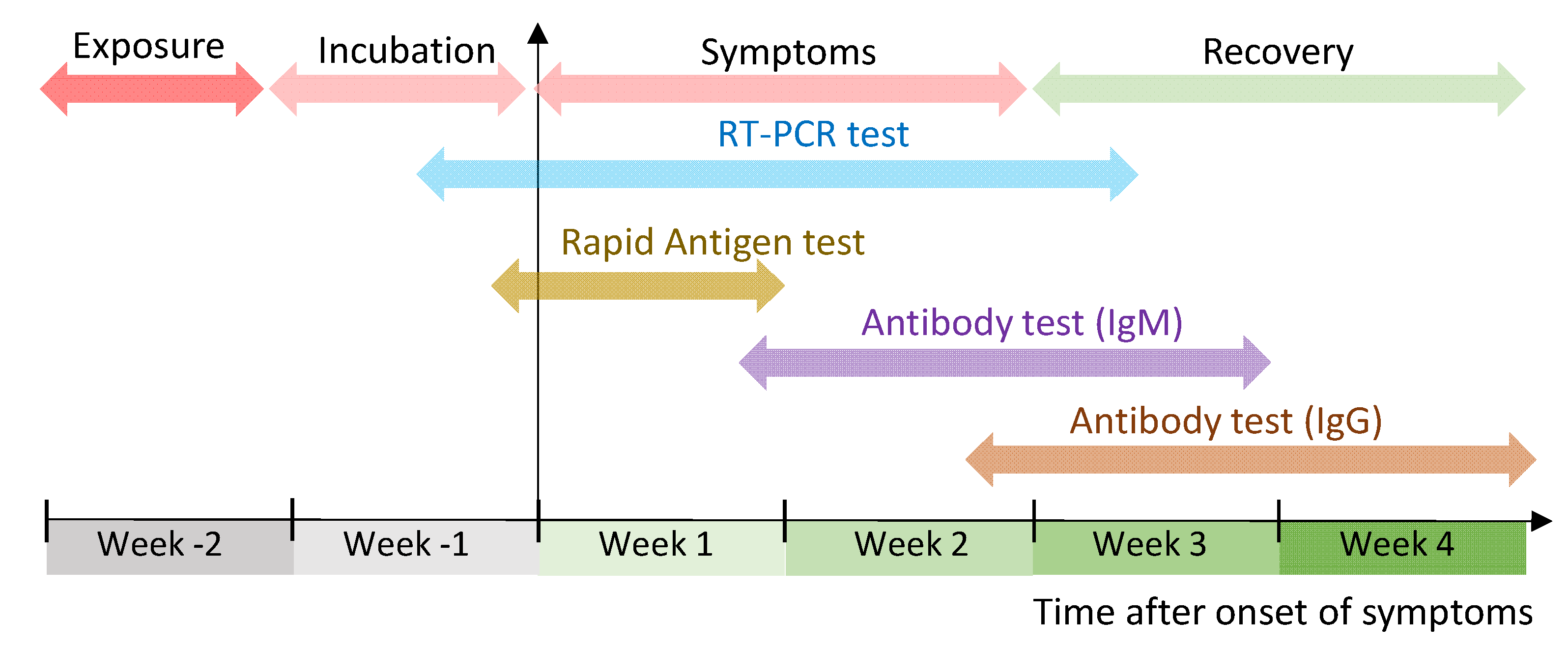
Quest Diagnostics Covid Antibody Test Fda Approval And Save Your money. October 8 2021. There are more than 70 COVID-19 antibody tests on the market because of the urgent situation but only four are approved under the FDAs emergency use authorization EUA process.
California health officials said many new covid-19 antibody test are being offered but only few are approved by the fda. Undergone the same type of review as an FDA -approved or cleared IVD. After reading this Fact.
The FDA said Saturday that it reissued an emergency use authorization to Quest Diagnostics to use its COVID-19 test with pooled samples. Quest LabCorp launch nationwide COVID-19 antibody testing in pharmacies and online Meanwhile Quest said it would work with the test developer Ortho Clinical Diagnostics to expand COVID. At this time researchers do not know whether the.
A positive test result for COVID -19 indicates that RNA. Visa MC AMEX eCheck Paypal Work time. The Quest test is the first COVID-19 diagnostic test to be authorized for use with pooled samples.
Sample pooling is an important public health tool because. SECAUCUS NJ April 21 2020 PRNewswire -- Quest Diagnostics NYSE. This limits a tests effectiveness for diagnosing COVID-19 and is why it should not be used as the sole basis to diagnose the disease.
Is Quest Covid 19 Antibody Test Fda Approved And Save Your money. Antibody tests or serology tests are blood tests that look for signs of an immune response to infection in this case immune molecules or antibodies specifically targeted to fighting SARS-CoV-2 the virus that causes the COVID-19 illness. FDA approves intravenous coronavirus antibody test Thomas Schinecker Roches head of diagnostics said the company aims to more than double production of tests.
The very best prices available today fast delivery. Quest Diagnostics is selling COVID-19 antibody tests online for 119. While we still dont know if these COVID-19 antibodies can protect you from being infected.
The serology antibody tests run by Quest Diagnostics are either approved by the FDA under an emergency use authorization or were released for use under the FDA guidance Policy for Diagnostic. Medical providers and testing capacity for the unfolding COVID-19 pandemic continues to be sorely tested. The FDA website lists authorized tests and tests offered under FDA COVID-19 diagnostic policy guidance.
The test will be administered at its nearly 2000 patient service centers more than 100 of which are at Walgreens locations as well as doctors offices. Go to your test appointment and then receive an email when your results are ready. It is the first test to be authorized to be used in this way.
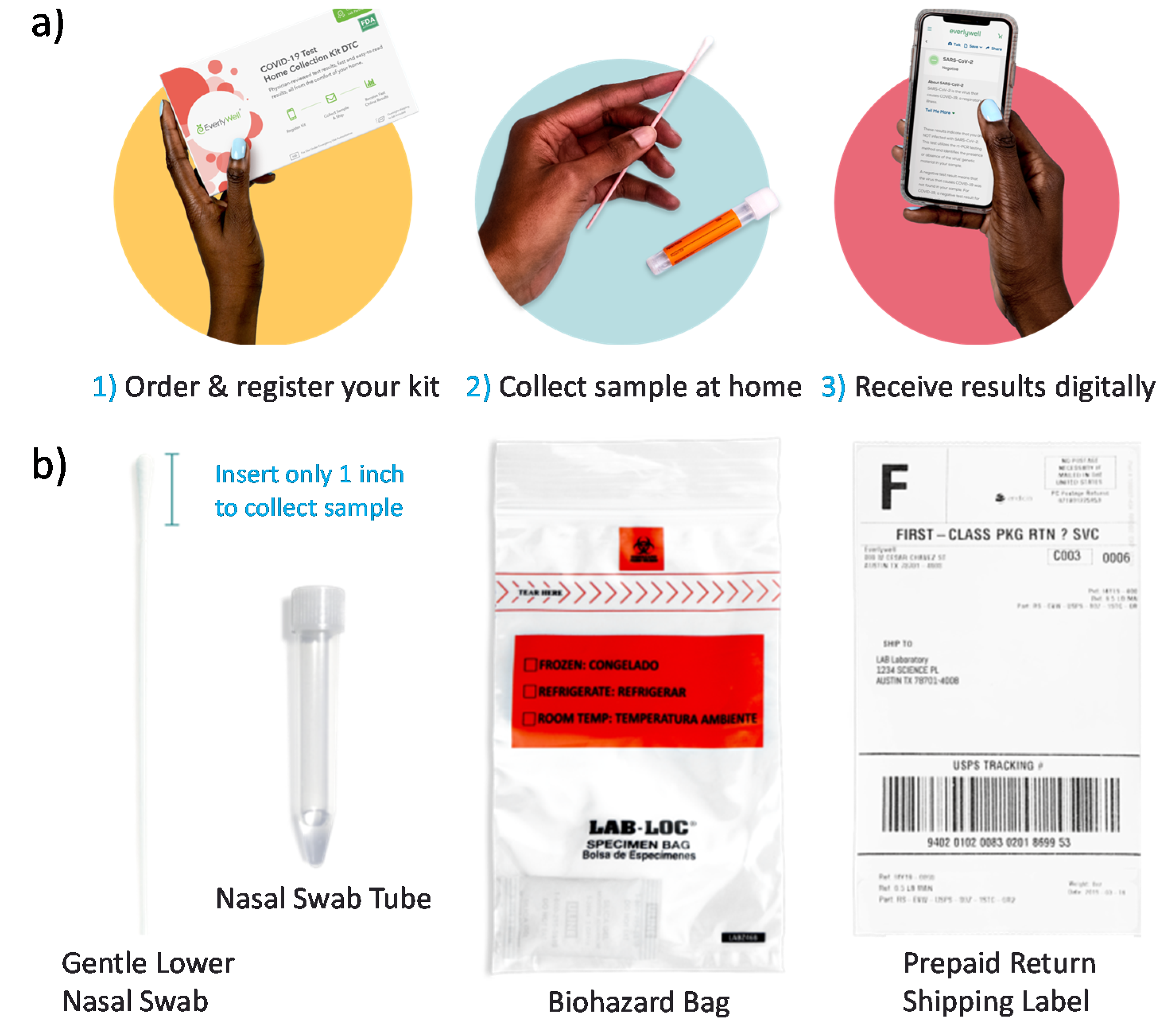
Convenient COVID-19 Testing How It Works. New antibody tests which are available in physicians offices urgent care centers and laboratories may provide some answers. COVID-19 using the Quest Diagnostics HA SARS - CoV-2 Assay on a anterior nasal swab collected using the Quest Diagnostics Collection Kit for COVID -19.
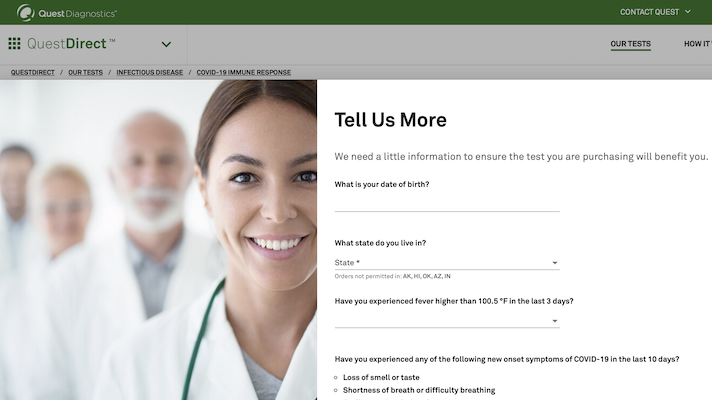
This Fact Sheet contains information to help you understand the risks and benefits of using this test for the diagnosis of COVID -19. Answer a few questions to see if youre eligible. The serology antibody tests run by Quest Diagnostics are either approved by the FDA under an emergency use authorization or were released for use under the FDA guidance Policy for Diagnostic.
The market has been flooded with options because the US. COVID-19 antibody tests can help identify people who may have been infected with the SARS-CoV-2 virus or have recovered from a COVID-19 infection. Antibodies may not be present in detectable levels in early days of an infection.
Quest S New Service Lets Customers Request Covid 19 Antibody Test Mobihealthnews

Was That Covid 19 Antibody Tests Promise Answers But Beware Of Their Limits Wbur News
Sensors Free Full Text Covid 19 Testing And Diagnostics A Review Of Commercialized Technologies For Cost Convenience And Quality Of Tests Html

Antibody Serology Tests For Covid 19 A Case Study Msphere

Covid 19 Antibody Test Questdirect



Tidak ada komentar:
Posting Komentar