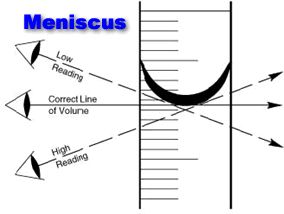
A meniscus is a phase boundary that has been curved because of surface tension. The meniscus of a liquid is the upward or downward curve seen at the top of a liquid in a container.
What Is A Meniscus Why Is It Necessary To Know About It When Measuring Liquids Socratic
Meniscus-guided coating MGC is one promising technique to tune the crystallization and thin film morphology of OSCs.
Importance of meniscus in chemistry. The meniscus of a liquid is the upward or downward curve seen at the top of a liquid in a container. The best way to do this would be kneeling down so your eyes are perpendicular with your measuring cylinder and measuring the bottom of the meniscus from there to avoid parallax errors. The meniscus also provides stability within the knee.
Updated October 02 2019. To have an accurate reading you must read from the bottom of the curve meniscus. However before we explain why some liquid have a concave up meniscus while others share a concave down meniscus we have to understand the adhesive forces at work of surface tension.
This phenomenon is important in transpirational pull in plants. A meniscus is the curved surface of a liquid in a container due to surface tensionThe measurement is at the bottom for a concave meniscus and at the top for a convex meniscus. In the case of water and most liquids the meniscus is concave.
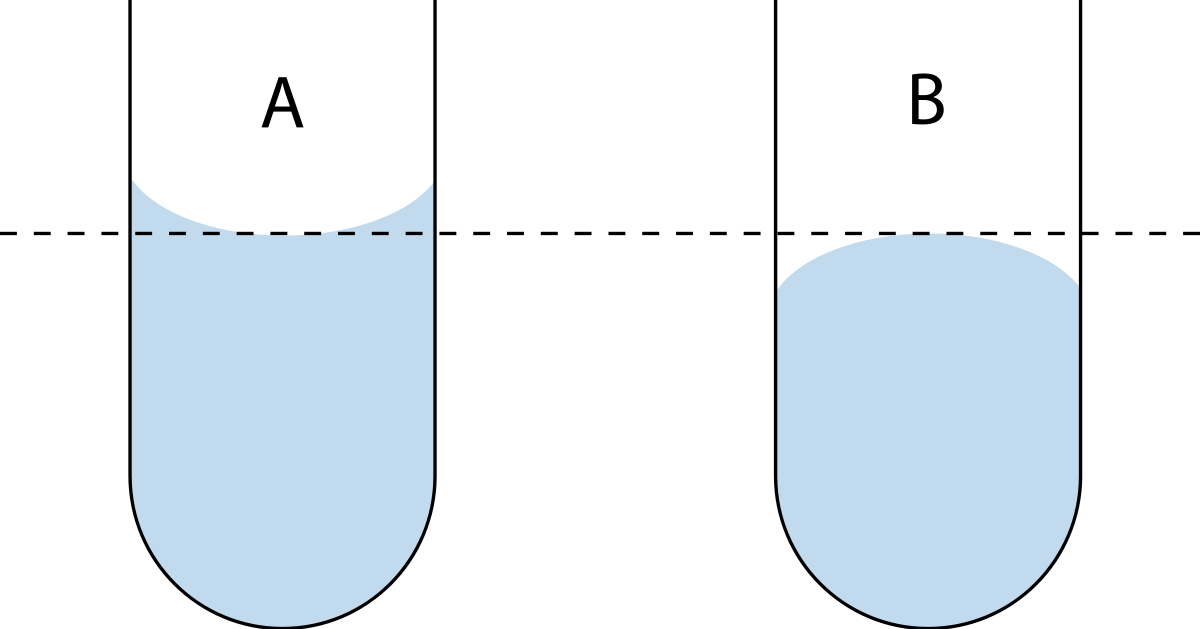
For a concave meniscus the correct volume will be read at the. There are two types of meniscus - concave and convex A concave meniscus occurs when the particles of liquid are more attracted to the container than to each other. Meniscus in Chemistry A concave meniscus forms when the liquid molecules are more attracted to the container via adhesion than to each other via cohesion.
The meniscus of a liquid is the upward or downward curve seen at the top of a liquid in a container. 40 40 Points What is the importance of the meniscus in this pipetting. In this work we demonstrate the crucial role of the meniscus shape in the fluid flow and crystallization of OSCs during MGC.
The shape of the meniscus is determined by the attraction of particles in the liquid to the walls of the container. A meniscus is a critical shock absorber within the knee. This example emphasizes the importance of that cohesive force and adhesive forces do not simply cancel each other out.
The meniscus is the curve seen at the top of a liquid in response to its container. A meniscus occurs because of surface tension in the liquid and must be read at eye level. The meniscus of a liquid is the upward or downward curve seen at the top of a liquid in a container.
The meniscus refers to the curved portion at the top of the liquid in the pipette that has to be taken into account to achieve an accurate measurement. The bottom of the curve is known as the meniscus and this point is what you should use to take your measurements. The meniscus plays two important roles within the knee.
How Important is the Meniscus. When the meniscus is torn and thus not functioning well it will lead to more stress on the cartilage on. If particles are more attracted to the container than to each other they will form a concave meniscus.
Menisci are a manifestation of capillary action by which either surface adhesion pulls a liquid up to form a concave meniscus or internal cohesion pulls the liquid down to form a convex meniscus. A narrow meniscus shape at small angle b elevates the evaporation rate n m and upward flow at the meniscus leading to a larger concentration gradient near the contact line favoring the crystal growth of the OSC during MGC. The meniscus can be either concave or convex depending on the surface tension of the liquid and its adhesion to the wall of the container.
Why a meniscus occurs. Why does a. With water you can think of it as when water sticks to the inside of a glass.
A meniscus is a critical shock absorber within the knee. A meniscus is a curve in the surface of a molecular substance water of course when it touches another material. Yes the meniscus is when water in say a measuring cylinder or burette curves up the sides of the glass.
For a concave meniscus the correct volume will be read at the bottom of the curve. A meniscus occurs because of surface tension in the liquid and must be read at eye level. This fluid flow assisted.
Again the meniscus is a very important structure. The nature of curve whether upward convex or downward concave depends on the surface tension the liquid and its adhesion capacity to the wall of the container. Adhesion is responsible for a meniscus and this has to do in part with waters fairly high surface tension.
What is a meniscus. Upward and recirculation flow is mainly affected by the meniscus shape. The meniscus also provides stability within the knee.
The meniscus plays two important roles within the knee. Water molecules are attracted. The nature of curve whether upward convex or downward concave depends on the surface tension the liquid and its adhesion capacity to the wall of the container.
A meniscus occur due to surface tension within a liquid. The meniscus is the curvature of a liquids surface within a container such as a graduated cylinder. The nature of curve whether upward convex or downward concave depends on the surface tension the liquid and its adhesion capacity to the wall of the container.
Why is a meniscus important in measurement.

What Is A Meniscus Definition Uses Lab Examples Video Lesson Transcript Study Com
/GettyImages-692027135-fdcc07eeb4604401beb7e020f0e5b310.jpg)
How To Read A Meniscus In Lab Measurements

What Is A Meniscus Definition Uses Lab Examples Video Lesson Transcript Study Com

What Is A Meniscus Definition Uses Lab Examples Video Lesson Transcript Study Com

Tidak ada komentar:
Posting Komentar